CCBBI MRI Safety Policies
Last Updated: 8/26/24

Table of Contents
- Contact Information for Safety Personnel
- fMRI Suite Access and Restriction Policy
- Safety Organization within the Center
- Information and Procedures Covered by the Safety Training
- Glossary
1. CONTACT INFORMATION FOR SAFETY PERSONNEL
Elizabeth Sponseller (MRI Technologist)
(614) 292-8911 (Office)
(330) 506-4739(Cell)
sponseller.21@osu.edu
Xiangrui Li (Assistant Director)
(614) 292-1847 (Office)
(626) 616-8528 (Cell)
li.2327@osu.edu
Ruchika Prakash (Director)
(614) 292-8462 (Office)
prakash.30@osu.edu
To Report an Emergency: dial 9-1-1
For non-emergency service call 292-2121
2. fMRI SUITE ACCESS AND RESTRICTION POLICY
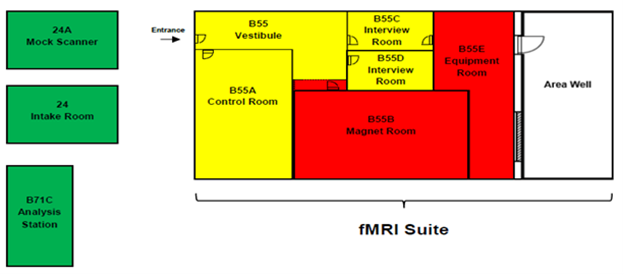
2.1 DIAGRAM OF THE MRI FACILITY
2.2 OVERVIEW OF THE SAFETY POLICY
The facility is divided into a number of “Safety Zones,” each with different rules and regulations that apply to that area (summarized in Figure 1 and below). Access to these zones is restricted according to a person’s “Safety Level,” which is determined by their degree of safety training and screening approval.
Safety Zones
Green: Intake Room, Hallway, mock scanner
- No Special restrictions
Yellow: control room and interview rooms
- Entry is denied if visitors do not complete the screening form
- Entry is denied to pregnant individuals unless they are here for a center-approved study
- Entry must be approved by Center staff
- Keep yellow zone door closed
- Keep area in front of magnet room door clear of metallic carts and equipment
Red: Magnet room and equipment room
- All yellow zone rules apply
- Entry is denied if having contraindications to magnetic field exposure (e.g., pacemaker)
- Personnel access requires MRI safety training
- No metallic objects/equipment allowed in the magnet room
- Personnel must be outside magnet room during MRI scan
- Must keep magnet room door closed
All visitors entering the Yellow or Red Zone must be screened for contraindications prior to entering the magnet room. Personnel will be asked to complete and sign an MRI Screening Form and undergo a screening interview with CCBBI staff, who will then make a decision about the individual’s suitability to enter a high magnetic field environment. If some contraindications are revealed during the screening process, the individual will be restricted from the Red Zone (magnet room) but may still be granted access to the Yellow Zone under the supervision of Center staff.
Metallic objects are not allowed in the magnet room. Metallic objects related to the experiment must be approved by Center director and MRI physicist during the protocol review.
Safety Levels
Unscreened Visitor
- Entry to Green Zone
- No screening required
- Must not enter fMRI Suite (Yellow/Red Zones)
Screened Visitor and/or screened human subject
- Entry to Yellow and Red Zones (w/supervision) for short-term visit
- Complete a Visitor Screening Form
- Pass a screening consultation and be approved by Center staff
- Only allowed entry to magnet room if accompanied by Center staff.
- Follow safety rules outlined in MRI Safety Checklist
- Obey safety instructions from Center staff or MRI operator
MRI User
- Access to Yellow Zone/Red Zone to support execution of MRI experiment
- Attend safety orientation given by staff and read the MRI Safety Training Manual
- Watch and sign off on MRI Safety Video
- Complete a Screening Form, Visitor Safety Checklist, and User Training Checklist
- Screened by Center staff if not already done so
- Follow safety rules outlined in MRI Safety Checklist and video
- Must be familiar with safety procedures outlined in the MRI safety manual, including quench and emergency procedures
- Notify Center staff if screening status changes
MRI Operator
- Authorized to operate MRI
- Must be trained in scanner operation
- Pass exams for safety and hands-on operation tests
- Must be certified by the Center Management Committee
- All MRI users are responsible for human subject
- Ensure safety of other uses and direct emergency procedures
- Lock door to magnet room and equipment room if last one to leave facility
- Run human subject with the supervision of Center staff
3. SAFETY ORGANIZATION WITHIN THE CENTER
The Center for Cognitive and Behavioral Brain Imaging (CCBBI) is a research-only unit. Clinical studies are not undertaken at the Center. With respect to safety, the Center’s activity falls under the general guidelines at the Ohio State University and other relevant policymaking bodies of the state and federal government.
The final responsibility for safety within the Center rests with the Management Committee, which comprises individuals with knowledge of MRI procedures, medicine, neuroscience, physiology, physics, and electronics. In the event that an unsafe condition arises, or if a safety policy has been violated, the Management Committee has the authority and responsibility to revoke approval of the protocol involved until the condition is corrected. The Committee and the MRI Physics Manager have this authority pro tempore.
The MRI Physics Manager is experienced and knowledgeable about the operation of the MRI scanner, safety hazards, and safety policies of the Center. The Physics Manager has the authority to suspend any activity at the Center that violates the safety policies of the Management Committee (or University) or that otherwise constitutes an unsafe condition.
The Physics Manager may transfer this authority to an approved operator of the facility. The Physics Manager will perform the following safety-related tasks:
- Ensure that the safety policies of the Management Committee are followed during the execution of approved MRI research protocols.
- Coordinate training classes concerning safety practices at the Center.
- Maintain a permanent file of incident reports and corrective actions taken.
- Ensure adequate distribution of the safety manual within the Center.
- Remain updated on new governmental and non-governmental policies and recommendations regarding MRI safety.
- Report employee accidents to the university safety department and to the Management Committee.
A qualified MRI operator will be responsible for performing all MRI procedures. He/she must have the following qualifications:
- Successfully completed a formal training course on safety led by the Physics Manager.
- Successfully completed hands-on MRI training at the Center under the supervision of an experienced MRI technologist.
- Successfully passed a comprehensive written examination and a hands-on operating test.
- Granted approval or certification by the Management Committee.
4. INFORMATION AND PROCEDURES COVERED BY THE SAFETY TRAINING
4.1 PERSONNEL CATEGORIES AND TRAINING REQUIREMENTS
4.1.1 PERSONNEL CATEGORIES
This section details policies and procedures to ensure the safe operation of the MRI research facility, to protect volunteers, to protect research personnel and staff and to safeguard the CCBBI infrastructure.
The CCBBI has a categorical scheme for those who enter the fMRI suite. The scheme has a hierarchical character with increasing levels of training and explicit permission required to use the facilities and equipment.
Volunteer: Individual who provides informed, written consent to participate in approved research protocols.
Visitor: Individual without any or incomplete training related to MR safety and human subject participation.
MRI-User: Individuals who have passed safety and basic equipment training to ensure one’s own safety during research-related activities within the MRI center. Those researchers involved with human participants will have also completed the human subject-
training regimen (CITI). Approved MRI-researchers can enter yellow zone unescorted, but they cannot escort Volunteers or Visitors into the magnet room (Red zone) without the explicit approval of an MRI-operator.
MRI-Operator: These individuals have passed MRI safety and equipment training to ensure safety of self and others during activities in the MRI center. They are also approved for participant screening (metal, pregnancy, etc.). They receive in-depth equipment training and knowledge of emergency procedures and are approved for independent operation of the 3T MRI system. The CCBBI MRI technologist oversees certification of MRI-Operator status and has the right to rescind this privilege at any time
4.1.2 TRAINING REQUIREMENT
MRI Safety Training: All personnel planning to enter the fMRI suite (yellow and red zones) for the purposes of conducting research must complete Basic MR Safety Training. Basic MR Safety training will be done on-site by CCBBI staff and consists of a presentation that includes viewing of a Siemens safety video. This format will give individuals a chance to ask questions and get answers to any concerns that they might have. Initial training also includes a familiarization with the facility. Once initial training is complete, yearly refresher courses may be done entirely on-line. The CCBBI administrative assistant will maintain a log of safety training for all MRI-researchers and operators.
Individuals seeking to become MRI-Operators must complete an Advanced Safety Training course. This is a more detailed coverage of safety procedures, human subject screening procedures and emergency procedures.
Emergency Procedures Training: MRI-Operators will receive instruction on procedures related to situations involving medical emergencies or those presenting an immediate threat to human life or to the facility infrastructure. This training is recommended, but it is not required for MRI-researchers.
MRI system operations: At least one Center staff member and one MRI operator or MRI user are required to be present for all MRI sessions in which there is a human subject. At the discretion of the CCBBI, certain MRI-Researchers may be trained and certified to operate the MRI system as an MRI-Operator. Only OSU-employed faculty, post-doctoral fellows and graduate students may be certified to operate the MRI system for research.
The CCBBI MRI technologist will conduct MRI system operation and related training.
Certification to operate the MRI system will be conferred by the CCBBI Director after examining the competency of the individual in question and based on the recommendation from the MRI technologist. Approval will be based upon completion of an oral examination and a hands-on exam. The training will involve onsite observation and supervised practice of the operational procedures of the MRI system and safety and emergency protocols. Anyone certified to operate the MRI system must also have received Emergency Procedures Training. When MRI-Researchers pass all training and examinations, their certification to operate the MRI system can be awarded by the Management Committee of CCBBI.
Renewal of MRI Safety Training: Annual hands-on refresher training will be required of all certified research personnel. Additional ad hoc training may occur due to newly developed safety guidelines.
4.1.3 TRAINING PROGRAM CONTENTS
Basic Safety Training
- Watch Siemens safety video
- Site specific orientation
- Location of scanner
- Instruction to attend to and obey all posted signs
- Emergency evacuation plan
- De-metaling
- Hearing protection
Emergency procedures
- Location and use of Emergency Electrical Power Shutdown buttons
- Location and use of Magnet Stop (Quench) buttons
- Medical Emergency procedure
- Quench procedure
Scanner Operations Training
- Human subject screening procedures
- Squeeze ball
- Human subject preparation
- Patient table controls
- Minimum 6 hours of shadowing CCBBI personnel performing magnet operations
- System start-up and shut-down procedure
- Routine scanning
- Patient table controls
- New human subject registration
- Protocol selection
- Prescribing protocol sequences and setting measurement parameters
- Data archival and retrieval
- Logging
- Human subject monitoring (intercom) system
- Incidental finding protocol
- Oxygen sensor location
- Coil handling and storage
- Linen storage and use
- Knowledge to access data on cryogen levels
- Knowledge of SAR and stimulation warnings
- Phantom placement and scanning
- Orientation for non-Research Personnel
- Non-research personnel (such as custodians and non-Center staff) who require routine access to scanner or equipment rooms (Red Zones) must receive an orientation and complete safety training
- Basic familiarity with the hazards associated with the magnetic field
- Missile effect
- Malfunction of implanted medical devices
- Familiarity with the layout of the suite
- Location and meaning of magnetic field
4.2 EMERGENCY PROCEDURES
4.2.1 MEDICAL EMERGENCIES
- In a medical emergency, the MRI-operator will instruct the second individual, who is required to be present for all human studies, to dial 9-1-1 to report the nature of the emergency along with the location to the response team.
- Emergency procedures shall NOT be administered in the magnet room, and NO medical equipment shall be allowed in the magnet room. Instead, the MRI operator or emergency team shall remove the human subject immediately from the magnet room and transport her/him to the interview rooms, where the emergency will be handled by the medical response team.
- The magnet room door shall be closed upon removal of the human subject to avoid entry of any metallic objects.
- If not present, the principal investigator shall be contacted and informed of the nature of the emergency.
- All adverse events shall be documented on an incident report. The IRB and the physics manager will be notified immediately via telephone and within 48 hours in writing.
4.2.2 FIRE EMERGENCIES
If an electrical fire were to occur in any of fMRI Suites (including the control, magnet, interview or equipment rooms), one nonferrous water mist fire extinguisher is located outside the magnet room door to contain the fire. Personnel are not required to fight fires and should evacuate the building immediately in the event of a fire.
- The MRI operator shall immediately remove the human subject from the magnet room. All personnel should evacuate the building according to the building evacuation plan, in a calm manner. Never use elevators.
- Disconnect electrical power to the MRI system by pressing the emergency ELECTRICAL SHUTDOWN button.
- If the fire occurs in the magnet room, the fire shall be extinguished using a non-ferrous fire extinguisher (the fire extinguisher near the outside door of the Center is non- ferrous).
- If the fire is not extinguished after emptying the available extinguisher, or jeopardizes the safety of personnel, the magnetic field must be removed by pressing the MAGNET STOP button on the Emergency Rundown Unit.
- All personnel, including firefighters, must be screened for entry into magnet room until magnetic field area is less than 5 Gauss.
- All doors shall be closed to contain the fire.
- 9-1-1 shall be dialed identifying the type and location of fire.
- Do not reenter the building until granted permission by the Fire Department.
4.2.3 QUENCH
The MRI magnet is maintained at a high field strength by means of super-cooling its conductive loops of wire with liquid helium, which is at an extremely low temperature – close to absolute zero (about 4°K). In certain circumstances, this helium may be rapidly vented off, warming the magnet and causing it to quickly lose its magnetic field either intentionally or unintentionally. This is known as a “quench.” A quench may be released by pressing the magnet’s “Emergency STOP” button (the one with a red “do not touch” label). This is referred to a “controlled quench”. Another source for quenching is a helium fill level that decreases to a point (about 30%) where the magnet begins to warm up. This is known as an “uncontrolled quench.” In rare instances, a spontaneous quench may be observed that cannot be explained by the presence of obvious external sources.
Intentional or Magnet Stop Quench
An intentional Magnet Stop quench should only be initiated by authorized personnel in the event of a potentially life-threatening emergency, such as an individual in respiratory distress being pinned to the magnet by a metallic object. A quench of the magnet is extremely expensive and has the potential to damage the equipment. An intentional quench is performed in an extreme emergency to rapidly run the magnetic field to zero. A quench of the magnet should only be performed when:
- A person is pinned to the magnet and is unable to free themselves without harm.
- There is a fire in the MRI scanner, equipment, or console room.
- There is a fire in another area that is a threat to the MRI Suite
In situations not posing a threat to human life, such as a piece of equipment being pinned against the magnet, no one should initiate a quench. If a metal object traps a human subject in the magnet bore so that removal is not possible without quenching the magnet, or if it is determined that a potentially life-threatening situation exists, then the operator or his designee should press one of the two Magnet Rundown buttons.
- The magnetic field will dissipate in approximately one minute.
- Use the intercom to alert the human subject to stay calm and remain on the table until the operator gains access to the room to offer assistance.
- If the quench was initiated because of a medical emergency, the procedures listed above under Medical Emergencies should be followed.
- After ensuring that the magnet and equipment rooms are secure and that all individuals have exited these areas, inform the director and notify Siemens of the quench.
- Secure the magnet room door. The MRI magnet operator is responsible to ensure that no one enters the magnet room without proper screening for MRI safety. Note that even in the event of a quench, a significant magnetic field may remain for some period of time.
- After a quench, the usual service procedure goes into effect (please refer to the Siemens System Manual located in the cabinet in the control room). Siemens Customer service must be notified as quickly as possible to put the system back into operation.
- File an incident report and notify appropriate University personnel. The following offices must be notified:
- Department of Public Safety.
- OSU Office of Environmental Health and Safety
- OSU Office of Insurance & Risk (if involving injury which may result in an insurance claim).
Uncontrolled Quench (or spontaneous quench)
It is possible, but highly unlikely, that a spontaneous quench of a magnet could be caused by an accident (earthquake, fire, etc.). If a spontaneous quench occurs, remove the human subject immediately and follow the same procedure as a user-induced quench.
4.2.4 EMERGENCY ELECTRICAL SHUTDOWN
The Emergency ELECTRICAL SHUTDOWN button is located on the wall in the MRI magnet room and on the wall in the control room. It removes ALL electrical power from the MRI console, the console computers and the patient table, including any power sources from Uninterrupted Power Supply (UPS) devices. Pushing the Emergency SHUTDOWN button turns off the entire MR system EXCEPT for the static magnetic field and the MAGNET RUNDOWN unit (described below). Pushing the Emergency SHUTDOWN DOES NOT PRODUCE A QUENCH. It does not turn off the lights. Also, power to the stimulation equipment will not be interrupted, so be aware that electrical or fire hazards may still be present. The button should be used ONLY TO STOP A SCAN DURING A HUMAN SUBJECT EMERGENCY or DURING A SERIOUS EQUIPMENT FAULT OR HAZARD, such as fire or water in the vicinity of the MR equipment.
4.2.5 EMERGENCY MAGNET RUNDOWN
The device for an Emergency Rundown, pictured below, allows for the rapid reduction of the magnetic field in about one minute. It will also boil-off cryogens and therefore, unlike the Emergency ELECTRICAL SHUTDOWN button, this button WILL PRODUCE A QUENCH. The button is located inside the magnet room on the left wall adjacent to the door. Only the MRI physics manager or director of the MRI Center is authorized to trigger the rundown UNLESS A HUMAN LIFE IS AT RISK (i.e. do not quench the magnet if a piece of furniture or equipment got into the bore. Such objects can be safely removed by calling a Siemens’ service engineer who will slowly power down the magnet). The rundown should be triggered to free someone pinned to the magnet, or to remove a large ferromagnetic object captured in the magnetic field when injury to the human subject is imminent.


4.2.6 PROCEDURE FOR POWER FAILURE
In the event of a power failure, if an MRI study is in progress, the human subject will first be removed from the bore manually. Simply pull the patient table out of the bore. Once human subject safety is secured, the MRI operator will return to the MRI suite and properly shutdown all of the computers (which should be receiving power from UPS), thus preventing corruption of the software on the MRI scanner. The MRI Center and ancillary systems will remain off until facilities operations Department notifies the MRI Physics Manager of adequate power return.
4.3 GENERAL SAFETY CONSIDERATIONS FOR MRI SCANNING 3.0 TESLA
This CCBBI’s Siemens Trio 3.0 Tesla MRI scanner has been approved by the Food and Drug Administration (FDA) for human and animal use. It will be used solely for research purposes that will involve the use of human subjects, as well as MRI phantoms (containers filled with gelatinous materials or chemicals).
4.3.1 GENERAL SAFETY PROCEDURES IN THE MRI CENTER
- All human subjects must be evaluated by the principal investigator, or designee, as to their physical and mental status before entering the MRI suite.
- All individuals must undergo screening for metallic objects and complete the appropriate screening form.
- All individuals with critically implanted magnetic objects (i.e., aneurysm clips, pacemakers etc.) will not be allowed in the room.
- All human subjects must be supervised at all times while in the fMRI suite.
- No human research will be performed within CCBBI without prior approval of the university’s IRB.
- All human subjects must sign an IRB-approved informed consent form before entering the magnet room.
- When the MRI scan is in progress, human subjects will be given a signaling device to hold so that he or she can alert the MRI Operator of any discomfort or emergency.
- All human subjects will remain in view of the technical personnel, either via direct eye contact or via a camera system, while the MRI scan is in progress.
- When scanning a human subject, another person must be in the control room in addition to the operator.
- No animals will be scanned in the Center without prior authorization.
4.3.2 MRI-SPECIFIC RISKS
The risks of MRI scanning can be classified into one of four categories, those associated with
- Acoustic Noise Levels
- Gradient or Time-varying Magnetic Fields
- Radiofrequency (RF) Magnetic Fields, and
- Static Magnetic Fields.
Acoustic Noise The acoustic noise associated with MRI imaging is related to the mechanical movement of the gradient coils during the scanning process.
FDA Guidelines: "The acoustic noise levels associated with the device must be shown to be below the level of concern established by pertinent Federal Regulatory or other recognized standards setting organizations. If the acoustic noise is not below the level of concern, the sponsor must recommend steps to reduce or alleviate the noise perceived by the human subject." Current FDA guidelines follow the regulations of the International Electrotechnical Commission (IEC) Standard 601-2-33, which stipulate that for MR equipment used in medicine, hearing protection is required when the system can produce acoustic sound levels above 99 dB and that the protection should be able to reduce noise levels to below 99 dB.
The FDA has approved systems for which noise levels have been quantified, ranging up to 105 dB RMS for scanners operating at field strengths of 1.5 Tesla. It is important to note that the static magnetic field strength is only one factor, and not necessarily the most important one, in determining acoustic noise. Among the factors listed above, the design and construction of the gradient coils plays a major role in the noise level that MRI scanning produces. Therefore, noise levels are not necessarily greater when scanning at 3.0 T compared with 1.5 T field strengths. It is nevertheless possible that, in some circumstances, our system could produce noise levels higher than 99 dB, as do many clinical systems operating at lower field strengths.
The acoustic noise levels perceived by human subjects when undergoing MRI examination in our 3.0 Tesla magnet constitutes a non-significant risk; specifically, our system will not be operated in a way that will present more noise to human subjects than is approved or recommended by the FDA.
Ensuring Safety from Acoustic Noise: As suggested by the FDA, we will take steps to reduce or alleviate the noise levels experienced by human subjects in this protocol. This will be accomplished by one of two methods:
- Use of disposable earplugs
- Use of acoustically shielded headsets
Peripheral Nerve Stimulation: The time-varying magnetic fields used in MRI can, in some instances, induce stimulation of peripheral nerves, thereby producing sensations such as 'twitching' or 'tingling.' In very rare instances, this nerve stimulation can be painful. Nerve stimulation is particularly likely when human subjects are physically positioned in a way that increases the likelihood of inducing stimulation, such as with hands clasped or arms folded. It should be noted that the parameter of interest here, dB/dt (the rate of change in the magnetic field per unit time), is not a function of the strength of the static magnetic field, so evaluating risk in a 3T MRI scanner involves the same considerations as evaluating other MRI systems operating at lower magnetic field strengths (i.e., the same issues apply to all the commercially available, FDA approved scanning systems). Thus, it is the gradient system only that needs to be evaluated to determine the risk of producing nerve stimulation.
FDA Guidelines: The FDA Guidance of 1995 was developed specifically to consider the fact that many clinical systems were capable of exceeding levels of dB/dt that could produce nerve stimulation. It was originally considered that a warning level should be implemented to guard against peripheral nerve stimulation, but the FDA finally concluded that: '... this warning level is not considered critical since there are no harmful effects associated with mild peripheral nerve stimulation’. The current guidelines therefore include monitoring procedures to help avoid painful peripheral nerve stimulation, and without specific dB/dt limitations.
Summary of Risks: The gradients used in our 3.0 Tesla MRI system will typically be operated at levels below those considered to be negligible according to FDA guidelines. Our system, like most commercially available, FDA-approved systems, does have the capacity to exceed this level, but it will include the same safeguards that are included in other FDA-approved clinical systems. Furthermore, policies and procedures will be implemented according to FDA guidelines to avoid the possibility of painful peripheral nerve stimulation. Therefore, in all circumstances the system will be operated in a way that poses a non-significant risk to the participant.
Ensuring Safety from Peripheral Nerve Stimulation
- All consent forms for studies that might induce peripheral nerve stimulation will provide this information.
- If the built-in stimulation monitor is bypassed by a user sequence, record of dB/dt value will also be included with the imaging data to help in an analysis of levels of peripheral nerve stimulation possibly perceived by human subjects.
- If the built-in stimulation monitor is bypassed by a user sequence, detailed calculations of the changes in magnetic field over time (of which the gradient system is capable) will be calculated, and conservative values will be selected as limits that will be used to determine when special additional monitoring is indicated. In these cases, monitoring procedures recommended by the FDA will be used.
- The gradient switching times and strengths will be monitored together with the routine assessment of all electrical components of the system.
- All MR operators will receive special training to prevent peripheral nerve stimulation.
- Before any scanning procedure that might stimulate peripheral nerves, an operator will:
- Inform the human subject that peripheral nerve stimulation may occur.
- Describe the nature of the sensation to the human subject.
- Instruct human subjects not to clasp their hands as this may create a conductive loop which will increase the possibility of stimulation.
- Maintain constant verbal contact with the human subject.
- Instruct human subjects to inform the MR operator if they experience discomfort or pain.
- Terminate the scan if the human subject complains of discomfort or pain.
- Complete a report of any incidents involving severe discomfort or pain, including a description of the associated circumstances (imaging parameters, dB/dt value, level of pain, etc.), and submit this report immediately both to the IRB and to the MRI Safety Committee.
Tissue Heating: MRI scanning induces some heating of body tissues. This specific absorption rate (SAR) that determines heating is the amount of radiofrequency (RF) energy deposited (typically by a coil or “helmet”-like apparatus placed over the human subject’s head) per unit volume of tissue per unit time. RF energy in MRI examinations is not a function of the strength of the static magnetic field. Rather, the Specific Absorption Rate (SAR) for RF radiation is related to the amplitude of RF power, the duration of the RF pulse, the type of RF coil used, the frequency of RF radiation, the resistivity of the tissue, the configuration of the anatomical region being examined, and several other parameters.
FDA Guidelines: "The following are levels of concern at which the reviewer shall exercise appropriate actions to ensure that the safety of the device is substantially equivalent to a predicate device: A) If SAR 0.4 watts per kilogram (W/kg) whole body; and if SAR 8.0 W/kg spatial peak in any 1 gram of tissue; and if SAR 3.2 W/kg averaged over the head: below level of concern. Or B) If exposure to radiofrequency magnetic fields is insufficient to produce a core temperature increase in excess of 1°C and localized heating to greater than 38°C in the head, 39°C in the trunk and 40°C in the extremities: below level of concern. The parameter SAR cited above must be shown to fall below either of the two levels of concern by presentation of valid scientific measurement or calculation evidence sufficient to demonstrate that SAR is of no concern."
It should be noted that this guideline is based on the calculation of a system that has no thermoregulatory response, and thus it is a very conservative estimate compared with the temperature change that would be experienced in any living human subject. Normal diurnal temperature variations in humans, for example, are about +/-1°C from the normal set point 37°C, and healthy people with normal thermoregulatory responses can easily dissipate any excess (or, in this instance, deposited) heat by increasing their peripheral blood flow or sweat rate. Thus, the heating effect of MRI with the SARs used in accord with these guidelines is extraordinarily unlikely to cause any acute effects in healthy human subjects.
Summary of Risks: Because all experiments performed on the 3.0 Tesla system will comply with FDA guidelines with regard to SAR, and because appropriate RF power safety checks are in place, the criterion for classification of NSR is satisfied.
Ensuring Safety from Tissue Heating Risks: The magnitude of temperature increase during MRI scanning is minimal. Increases are always within FDA guidelines, which include core temperature increases less than 1°C, as well as localized heating to less than 38°C in the head, 39°C in the trunk, and 40 °C in the extremities. Our 3.0 Tesla system has in place a means to monitor RF power levels and ensure that energy deposition is sufficiently low to stay well within these guidelines for temperature increases. First, a "system security" unit is employed to integrate the output of the RF amplifiers. This integration takes into account the amplitudes and duty cycle of the transmitter. If system security detects an output that might exceed the guidelines noted above, it automatically shuts down the entire RF power system. Secondly, all pulse sequences are evaluated (based on calculations and sound scientific measurements) to ensure that SAR remains within FDA-approved guidelines prior to their use in humans. Any experiment performed on our 3.0 Tesla system will comply with all FDA guidelines with regard to RF power deposition. Proper and routine monitoring of all RF electronics (e.g., coils, transmitters, system security, etc.) will be performed on a regular basis. All pulse sequences will be evaluated (by calculation and by valid scientific measurement) prior to use in humans.
Static Magnetic Fields: The possible risks of static magnetic fields have received much attention in the lay press, but scientific consensus on these risks has yet to be fully reached. The FDA has deemed that systems operating at 8.0 Tesla or less do not pose a significant risk. Moreover, experience with thousands of clinical studies over the past decade, and with multiple human investigations carried out at higher field strengths over this period, have not revealed risks of exposure to higher static magnetic fields. The most significant risk associated with static magnetic fields is that a ferromagnetic object(s), such as aneurysm clips or a heart valve, can interact with the magnetic field of an MRI scanner, causing the device to malfunction and injuring the human subject. For some human subjects, rapid head movement while in the magnetic field may cause dizziness, vertigo, or a metallic taste in their mouth.
FDA Guidelines: The “FDA believes that a magnetic resonance diagnostic device used under any of the operating conditions… is a significant risk device as defined in 21 CFR 812.3(m)(4),” specific operating conditions include state magnetic fields greater than 8T.
Summary of Risks: This category of risk applies to work conducted around superconducting magnets of any kind (including standard clinical diagnostic MRI units). It is not unique to our 3.0 Tesla facility. The MRI facility will maintain a safety policy to safeguard human subjects and staff members from these incidental risks. Systems with static magnetic field less than 8 Tesla have been considered to represent a non-significant risk (NSR) by the FDA. The static magnetic field of our system (3.0 Tesla) is therefore to be classified as NSR with respect to human subjects.
Ensuring Safety from Static Magnetic Field Risks: The minimization of risks associated with the static magnetic field of 3.0 Tesla is mainly related to incidental risks (see below). These risks are the same as in other commercially available clinical systems, and like other clinical MRI centers, our facility will incorporate a complete range of procedures, including:
- Assuring the security of the restricted access area. Entrance doors to the MRI department will be kept closed at all times. Access to the MRI suite will be tightly controlled, allowing access for only personnel and human subjects who have legitimate reason to be there. Doors to the MRI suite will be securely locked.
- Entryways to the MRI suite will be labeled with clear visible signs warning of the presence of the magnetic field and the exclusion from entry by individuals with implanted metal objects such as prostheses, pins, clips, IUD’s, pacemakers, etc.
- The MRI operator will conduct careful screening of potential human subjects before they enter the magnet room (appended at the end of this document).
- To minimize the potential for dizziness or a metallic taste, it is recommended that the human subject remain still while in the region of the high static magnetic field.
Incidental Risks: The physical confinement and isolation produced by the scanner could cause emotional distress, although in our experience, human subjects have generally tolerated the procedures remarkably well.
All human subjects will be able to communicate directly with the operators to inform them of any emotional or physical distress during the scanning procedure. If they wish, the scan will be terminated immediately and the human subject will be removed from the scanner.
Ensuring Data Safety: All MRI data will be stored behind firewalls at CCBBI in accordance with university data policies.
Ensuring Documents Safety: Records regarding MRI safety and compliance, human subjects, scans, equipment maintenance and repair, as well as usage, are maintained by the CCBBI staff and the PI overseeing the research study. A list of records is outlined below:
Training Records: maintains safety and compliance training records for all personnel. The MRI Physicist Manager manages these records and maintains documentation of proficiency testing and copies of certification for MRI Operators.
Screening Forms: Preliminary safety screening forms for human subjects are kept on file by the PI overseeing the study. The second screening form for each subject is kept on file by the MRI Technologist. The MRI Technologist also maintains safety screening forms for MRI Operators, other OSU personnel, and visitors who enter the scanner room with human subjects.
Visitor Forms: Visitor Forms for tours of the MRI Suite are kept on file in the MRI control room and maintained by the MRI Technologist.
Consent Forms: Signed consent forms for each human subject involved in a study are maintained by the PI in accordance with IRB requirements.
Incidental Findings: The MRI Technologist maintains the MRI Incidental Findings Review forms. These forms do not contain identifying information and will follow the naming convention for scanner files. These forms are kept in a locked file cabinet in the MRI Control Room.
Data: The naming convention for all imaging studies will not contain any identifying information and will be listed as follows:
PI Name-Name of Study-Subject#
Per the rules and regulations of the OSU IRB, it is the jurisdiction and responsibility of the PI to keep their human subject volunteer information protected and confidential. They will retain copies of their own volunteer’s signed informed consents and assents, MRI prescreening, and any other documentation related to participation in their study. Once imaging data has been shared with the PI, it becomes their jurisdiction and responsibility to maintain and use the data in a confidential and appropriate manner.
Data Logs: The following logs are kept by the MRI Physicist Manager, Facility Manager, and MRI Technologist:
- Quality assurance data.
- Weekly temperature and humidity readings for the scanner and equipment rooms.
- Weekly cryogen readings.
- Scanner and equipment room filter change dates.
- Scanner communication log with Siemens for maintenance and scanner errors.
- IP addresses, port numbers, and application entry titles.
- Human subject archive log of all participants scanned.
Usage Logs: Accurate records regarding use of the scanner are required for proper billing and reporting to federal funding agencies. These records are reviewed and maintained by the CCBBI staff. When using the scanner, MRI Operators must record the following information:
- Date.
- IRB number (when appropriate) and study name or description.
- PI overseeing the project or study.
- Type of project or study (pilot study, human subject, phantom scanning, validation testing, etc.).
- Human subject number (when appropriate).
- Funding or Org number (ChartField).
- Start and end time of scanner use.
4.4 SPECIFIC HAZARDS WITHIN THE MRI CENTER
4.4.1 ELECTRICAL HAZARDS
- The MRI scanner will be evaluated regularly for electrical hazards by the Siemens Engineer as detailed in the service agreements with the MR system manufacturer.
- All modifications to the equipment will be performed only by the Siemens Engineer and will be properly evaluated in terms of electrical safety.
- Safety tests will be carried out on a regular basis by the Siemens Engineer with regard to radiofrequency and magnetic field levels, as detailed in the service agreements with the MR system manufacturers. After safety tests are completed, the service engineer will provide a comprehensive service report relating all results and action taken to restore any faults in MRI system.
4.4.2 CRYOGEN HAZARDS
A superconductive magnet in the MRI scanner uses cryogens to supercool the electrical conductor that creates the magnetic field. Temperatures as low as -269°C (-452°F) are achieved to create the proper superconducting environment within the magnet. A quench, which is a sudden boil-off of the entire volume of cryogenic liquid, causes a rapid loss of the static magnetic field.
Cryogens come in large vacuum containers called “Dewars”. Liquid helium is generally used for cooling purposes, although some service procedures also require liquid nitrogen.
Nitrogen Dewars weigh from 400 to 500 pounds when full. Helium Dewars weigh from 700 to 800 pounds. In addition to large Dewars, there may be smaller helium gas cylinders present. This helium gas is used to fill the magnet to the correct cryogen levels. The cryogens boil off as they cool the magnet wires and must be replenished periodically by qualified personnel. Contact with the cryogenic liquids or gas could result in severe frostbite, and care is needed when in proximity to these substances. Furthermore, leaking helium or nitrogen gas will displace oxygen from the room. An ambient air oxygen concentration of less than 17% to 18% is not sufficient for human respiration, and therefore a large cryogen leak or quench of the magnet is dangerous to humans in the room.
Safety Procedures:
- All dewars and gas cylinders must be non-magnetic.
- Dewars should be stored in a well-ventilated area.
- Gas cylinders should be stored upright and secured to the wall with a chain with a metal protective cap in place (if the cylinder falls over or the valve is knocked off, the container may act like a rocket as a full cylinder has enough power to penetrate walls).
- The valves of Dewars and cylinders should not be tampered.
- Because cylinder caps may be metal, they should be removed before bringing the cylinder into the magnet room.
- If possible, all personnel should stay out of the magnet room when a qualified service engineer is filling cryogens in the magnet. If personnel from the MRI suite must be present, they must wear proper gloves, a face shield, and ear protectors.
- Flammable material must not be brought near the cryogen containers.
- The wearing of protective clothing is essential during all work performed with liquefied cryogens. Such clothing consists of:
- Safety gloves
- Work gloves
- Face shield
- Laboratory coat or overalls (cotton or linen)
- Non-magnetic safety shoes
4.4.3 FIRE HAZARDS
General Safety Procedures:
- Necessary equipment (such as fire extinguishers) will be stored within the fMRI suite to manage all classes of fire. All equipment will be non-magnetic.
- To protect against the possibility of fire, flammable liquids in excess of five gallons will not be brought into the MRI suite.
Fire with Operators On-Site:
- The Operator will know all of the fire emergency related procedures, including a human subject evacuation plan, and its proper execution.
- The South Emergency exit located on the ground floor has been assigned as the point of exit for evacuation during a fire.
- The MRI Physics Manager or the Director of the Center will evaluate the need for an emergency quench of the magnet. If the situation is life-threatening, the MRI-Operator can quench the magnet.
- In the event of a fire requiring outside response, the MRI Physics Manager or the Director will quench the magnet if ferromagnetic equipment must enter the MRI magnet room. They will control entry and exit to the magnet room until the magnetic field is less than 5 Gauss.
Fire During Off Hours or No Operators On-Site:
- Contact the Director, the MRI Physics Manager, or other CCBBI staff immediately, the phone and cell numbers are at the front of this manual.
- Once contacted, the Director, MRI Physics Manager, or other CCBBI staff will instruct fire-fighting personnel and security staff as to the means of entry to the fMRI suite and to the proper means of quenching the magnet, if necessary.
- For purposes of access in an emergency, the OSU Department of Public Safety will have access to the fMRI suite.
4.4.4 INFECTION CONTROL PROCEDURES FOR HUMAN STUDIES
- Hands must be washed between human subjects.
- The MRI table and head rest must be covered using exam paper sheets. Sheets must be discarded after each human subject.
- All contaminated products must be discarded in the gray trash bin.
- The magnet room table, headrest, and padding must be wiped with a Sani-wipe at the end of the day.
4.4.5 SAFETY PROCEDURES FOR EXPERIMENTS WITH CONTRAST AGENTS
- Specific IRB approval for using contrast agents in an MRI scan must be obtained prior to the study commencing.
- The IRB proposal, consent form, and a scan record must include indications for use, recommended dose levels, warnings, and contraindications which are identified on the product inserts.
- Contrast agents can only be administered by a nurse under the direction of a specific physician or by the physician himself.
4.4.6 SAFETY PROCEDURES FOR EXPERIMENTS INVOLVING CHILDREN
- Specific IRB approval and consent forms for using infants or children in an MRI scan must be obtained prior to the scan.
- The child must be accompanied by a parent or an adult representative. The scans must be approved by the parent or the adult representative.
- When subjects are children, a careful explanation of consent forms and MRI scans is of the utmost importance.
- Children must be accompanied at all times and will not be allowed to move freely in the control room and magnet room.
- Children will be instructed that they will need to remain motionless for the duration of the scan.
- If a child cannot lie still without sedation, no scan will be performed. CCBBI does not use sedation.
4.4.7 SAFETY OF SECURITY PERSONNEL
- Security staff will have access to the magnet room within the Center only under the supervision of CCBBI staff.
- In the event of an emergency, the Security Officer will have on file a telephone number for the Center's Director, Associate Director, and MRI Physics Manager. Once contacted, the Physics Manager or the Co-director will advise the Security Officer on safe methods to access the facility and the safety procedures to follow once the restricted area is entered.
4.4.8 INCIDENT REPORTS
It is the duty of the MRI Physics Manager to report all violations of safety procedure and/or accidents to the Management Committee. The MRI Operator will document any of the following incidents in writing and immediately submit this report to the Physics Manager. This includes:
- Incidents in which a person was injured.
- Incidents requiring the emergency quench of the magnet.
- Incidents involving damage or potential damage to MRI and ancillary equipment.
- Conditions that constitute a safety hazard.
- Incidents in which an approved protocol was not followed, causing an unsafe condition.
Nothing in the foregoing is to be interpreted as preempting the legal and institutional responsibilities of the University’s Institutional Review Board or regulations of The Ohio State University.
4.5 SUMMARY OF SAFETY GUIDELINES
- Manuals
- The MRI Operator’s Manual and CCBBI safety manual should be in the control room whenever there is a human subject.
- Certification
- All console operators must complete the Center’s operator training and safety courses and updates.
- To maintain active certification, an operator must maintain safety training and pass safety and operation examinations.
- Research assistants who are not operators, but are essential to the running of a research study, may enter the scan room if they have successfully completed the required safety training (including any subsequent updates).
- Line of Authority
- Everyone in the fMRI suite has the responsibility of ensuring safety.
- The Certified-Operator running the console has the ultimate AUTHORITY to enforce safety standards. If the operator runs a human subject, the operation must be supervised by at least one CCBBI staff.
- No one may enter fMRI suite (yellow and red zones) without approval from CCBBI staff.
- No one may enter the magnet room (red zone) unless they are being actively supervised by CCBBI staff.
- Human Subject Policies
- IRB approval for each study must be obtained prior to conducting research with human subjects.
- Individuals who are or may be pregnant are not allowed to remain in the MR scanner room while the RF and gradients are operating, unless specific approval is obtained from the IRB for a center approved study.
- Human subjects may be scanned only after the investigator has obtained IRB approval for the study.
- At least one CCBBI staff member must be on site when a human subject is being scanned. NOTE: the human subject may not count as one of the MRI safety trained individuals.
- Human subjects may not enter the magnet room with metal.
- Human subjects with whom no reliable communication can be maintained may not be scanned at the center.
- Screening
- Anybody working within the magnetic environment (yellow and red zones) must be screened for safety risks prior to entering the magnetic field. This includes individuals who may be accompanying a human subject. THERE ARE NO EXCEPTIONS TO THIS POLICY.
- Human subjects must be screened for the presence of implanted or attached medical devices or other objects.
- The screening must be approved prior to each time a subject enters magnetic environment.
- Only a CCBBI staff will perform the screening.
- Any human subject with diminished cognitive abilities must be screened with a hand-held metal detector to determine if they have overlooked metal on their body.
- Individuals who screen positive for implanted or attached medical devices must not be allowed into the magnet room unless prior approval is obtained from the Physics Manager and/or the Center Director.
- For individuals who screen positive for implanted or attached medical devices, the name of the device, its manufacturer, and its part number is required. Only after the device is identified, documentation of its MR compatibility and safety is secured, will they be permitted into magnet room.
- Anybody working within the magnetic environment (yellow and red zones) must be screened for safety risks prior to entering the magnetic field. This includes individuals who may be accompanying a human subject. THERE ARE NO EXCEPTIONS TO THIS POLICY.
- Devices
- The CCBBI staff must review and/or test the safety of any behavioral or physiological devices entering the magnet room.
- Tested devices must be positioned in the scan room BEFORE any human subjects are in the vicinity of the magnet or positioned on magnet table.
- Special care will be given to positioning of any wires attached to the device. Wires must not touch the research subject. Wires should be kept straight and not contain loops.
- Remove unused transmit or receive coils from magnet bore and bed.
- Emergencies
- Medical Emergency: In case of a human subject with a medical emergency including illness or injury: 1) The human subject must be assisted out of the magnet room. 2) Operator dials 9-1-1 for assistance.
- Emergency Stop: If there is an emergency such as an equipment failure that could cause injury; sparking of equipment or a fire, the scanner operator or designee must immediately perform an Emergency SHUTDOWN.
- Magnet Emergency:
- If a human subject is restrained or pinned by a ferrous object to the magnet:
- Assess if the situation is life threatening, if YES a MAGNET RUNDOWN to quench magnet can be performed by an authorized personnel.
- If a human subject is restrained by a ferrous object to the magnet and is NOT in a life-threatening situation, call a Siemens engineer for assistance to determine the optimal way of releasing the human subject from the magnetic field. If a quench is necessary, proceed as above.
- Report the incident as an accident after procedure.
- If a human subject is restrained or pinned by a ferrous object to the magnet:
- Emergency Quench: A quench includes the rapid release of cryogens and results in the loss or significant decrease of the magnetic field. A quench should ONLY be performed by authorized personnel in a dire emergency that involves serious personal injury or a life-threatening situation. Note: In the event of extraordinary circumstances (such as an earthquake or explosion) that result in an uncontrolled quench, the oxygen level in the magnet room may significantly decrease—possibly making breathing difficult.
- Visitors
- Visitors are not permitted inside the magnet room without the permission of the Center Director
- All visitors must be supervised by CCBBI staff.
- Peripheral Nerve Stimulation and other Sensory Effects
- If the human subject complains of pain or discomfort, including headache, stop the scan immediately. If the pain is not due to a biomechanical cause (awkward placement of body, poor placement of neck or head supports, etc.), terminate the study. If the discomfort is due to a biomechanical cause, the study may continue if the cause is corrected.
- If the human subject complains of tingling, a light touch sensation, or muscle twitching, stop the scan and assess the extent of the discomfort. If the discomfort cannot be minimized, the study must be terminated.
- Report complaints of unexplained discomfort or pain immediately to the MRI Physics Manager and the Center Director after the subject has been removed from the scanner room.
- When giving informed consent and, again, prior to entry into the magnet room, human subjects will be informed of the possibility that they should report to the scan operator excessive warmth, visual flashes, dots, scintillations, or tactile sensations during an MR study.
- Acoustic Noise
- All individuals entering the magnet bore must be provided adequate sound protection. Earplugs and headphones can attain this standard (< 99 db of audible noise).
- When earplugs are used to provide sound protection, only center-approved earplugs should be used.
- Dispose of earplugs after each subject is scanned.
- Radio Frequency Fields
- Each subject’s weight must be entered into the appropriate program field before starting a scanning session. This is a critical safety rule.
- The scanner fan should be ON at all times to maintain adequate airflow through the bore.
- The Console Operator should inquire whether the subject is too warm or cold periodically during the study.
- Use non-conducting pads when needed. Position the subject’s hands to the side and ensure that legs are not crossed.
- When using surface coils place a sheet or pillowcase between the coil and the human subject’s skin.
- Removable eye makeup must be washed off prior to a scan.
- Human subjects with permanent eyeliner or other metallic ink tattoos may be scanned only after they sign the informed consent document associated with a specific, approved IRB protocol. Such subjects must be informed of the risk of skin irritation. Maintain close communication with these subjects during the course of the scan.
- Do not interfere with the performance of the RF power monitor.
- Head studies producing SAR values greater than 3.2 Watts/kg must be approved by MRI Physics Manager, Center Director, and have IRB approval.
- Training Requirements
- MRI Safety Training: All personnel requiring access to the MRI facility for research and/or other activities must complete MRI safety training appropriate to their role in the work.
- Human Subjects Training: All researchers having a protocol that involves human subjects are required to have completed the CITI training.
- Emergency Procedures Training: For all protocols involving human subjects, at least two operators, who have completed MRI emergency procedures training and are capable of handling the MRI system operator in the event of an emergency, must be present during each MRI data acquisition session.
- MRF SAFETY ZONES
- The MRI suite is divided into three safety zones as indicated on the attached fMRI Suite (above, figure 1). These zones are different colors with each zone representing a progressively greater level of access restriction.
- Zone 1 (Green): Unrestricted areas for safety issues. However, all users must follow the rules for basement access. Zone 1 includes
- The wait-entry area of the fMRI Suite and data analysis Suite (Room B71)
- Mock Scanner Room (Room 24 A)
- Zone 2 (Yellow Zone): Highly restricted area (magnetic field < 5 gauss). All visitors and human subjects in Zone 2 must complete an appropriate screening, and be escorted by CCBBI staff to enter this zone.
- 3T Control Room (B55 A)
- Interview Rooms (B55 C,D)
- Zone 3(Red Zone): Exclusion area, potentially hazardous zone (magnetic field > 5 gauss). All persons entering Zone 3, including researchers, human subjects and special visitors must complete screening and sign an appropriate screening form. Nobody is permitted in any Zone 3 area unless accompanied by CCBBI staff.
- 3T MRI Scanner Room (B55 B)
- Equipment room (B55 E)
5. GLOSSARY
Cryogen: A substance for obtaining low temperatures; a refrigerant.
Exclusion Zone: The magnet room is considered the exclusion zone. All ferrous equipment must remain outside exclusion zone.
Ferromagnetic vs Ferrous: A ferromagnetic substance is one that has a large positive magnetic susceptibility (e.g. iron). Ferrous items can possess intrinsic magnetic fields and react strongly in an applied magnetic field. (Iron, Nickel, Cobalt).
Peripheral Nerve Stimulation: Sensations such as 'twitching' or 'tingling', usually in an arm or leg. In very rare instances, this nerve stimulation can be painful.
Quench: Quench is the term used to describe a rapid loss of field strength in a superconducting magnet. During a quench, the magnetic current dissipates as heat and causes the liquid Helium to boil off in gaseous form. MRI installations are designed with ventilation systems to handle the rapid boil off of liquid helium.
Restricted Access Area: All of the MRI Unit except the human subject waiting area.
Safety Zone Definition: there are four different safety zones suggested around the MRI scanner (Zones I-IV). The access to these zones as restricted in MRI facilities is defined by its purpose and distance from the MRI scanner
Static Magnetic Field: Static magnetic fields are measured in Gauss (G) or Tesla (T) with 10,000 G being equal to 1 T. According to the most recent recommendations and guidelines provided by the United States Food and Drug Administration (FDA), clinical MR systems are permitted to function on a routine clinical basis at static magnetic field strengths of up to 4.0 T and on research basis up to 11.4 T.
Tissue Heating: Heating of body tissues as a result of MRI scan.
Unrestricted Area: The unrestricted area of the MRI suite is the human subject waiting room.

