Last updated: 9/21/22
Appendix VII - CCBBI Access Policies
Levels of Access and Requirements
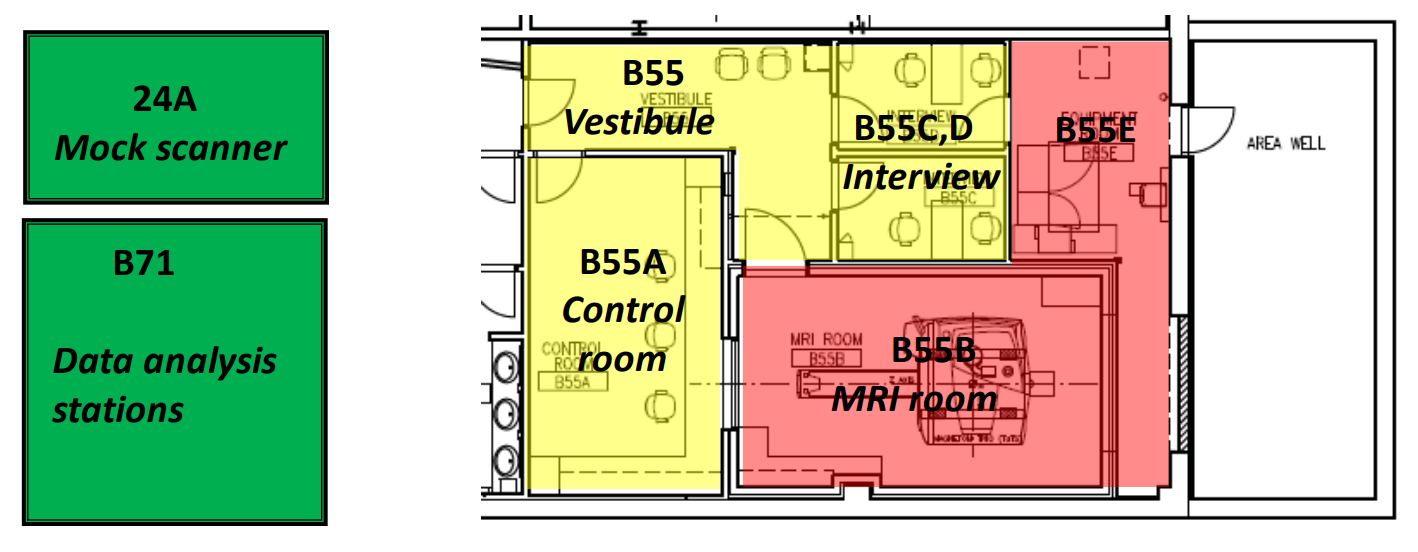
The accessible zones of the Center for Cognitive and Behavioral Brain Imaging (CCBBI) are labeled in different colors in the diagram below. The green zone includes the mock scanner room (24A) and the data analysis workstations (B71). The yellow zone includes vestibule (B55), two interview rooms (B55C and B55D), and the control room (B55A). The red zone includes the MRI room (B55B) and the equipment room (B55E).
Level 1 access is limited to the green zone only and is granted to relevant personnel of any approved and on-going study at the CCBBI for the duration of the study as designated by the PI.
Level 2 access covers the yellow zones. In addition to the requirement for Level 1, the additional requirements for Level 2 access are that the person must complete and sign either a visitor screening form or MRI screening form and pass a screening interview with CCBBI staff. Level 2 access is granted only between 8:00AM and 5:00PM on workdays. Entry to the yellow zones must be approved by Center staff. Off-hour access must be approved by the Director of the CCBBI.
Level 3 access covers the red zone. Persons granted access to the red zone must be accompanied by CCBBI staff.
Other Requirements for All-Level Access
All card-key holders must sign a statement acknowledging that entries to the CCBBI are logged and recorded, and those who are in the CCBBI can be held responsible for events occurring during their presence.
Users must sign in and out during off-hours.
Since accessing the CCBBI involves basement access, all users must follow the rules for basement access when in those areas.
Access Revocation
In the event that a person violates the access policy, the person will receive a warning for the violation and may have their access revoked. Repeated violations will automatically result in access revocation.
Access Renewal
All accessibilities are subjected to annual renewal. Unless otherwise stated, all keys are set to expire on 9/30 each year. PIs and/or supervisors will be notified with a list of personnel under their supervision three months prior to the expiration date. The Physics Manager or their designee will be responsible for this notification process.
Key access will be automatically renewed on the expiration date only if the following prerequisites are met prior to the expiration date: i) approval by the PI for an on-going study or by the CCBBI staff (Level 1), ii) completion of a refresher class on safety (Level 2), and iii) for certified scanner operators, completion of the annual re-certification process (Level 3).
Questions?
Please refer to the MRI Safety Manual for references and additional information. If you have any questions regarding accessibility, please contact us at ccbbi.service@osu.edu.
